OPTICAL REVIEW(Vol.12,No.3 May/June,2005)
Photodynamic Therapy for Cancer Cells Using a Flash
Wave Light Xenon Lamp.
Makoto Kimura 1,2, Satomi Yokoi 1, Yumiko Koiwa 1, Kasumi Kashikura 1,
Yoshikazu Tokuoka 1, ※ and Norimichi Kawashima 1,3
1 Faculty of Engineering, Toin University of Yokohama, 1614 Kurogane-cho, Aoba-ku, Yokohama 225-8502, Japan
2 Ushio Inc, 2-6-1 Asahi Tokai-Building 20F, Otemachi, Chiyoda-ku, Tokyo150-0021, Japan
3 Biomedical Engineering Center, Toin University of Yokohama, 1614 Kurogane-cho, Aoba-ku, Yokohama 225-8502, Japan
*To whom correspondence should be addressed. E-mail address: tokuoka@cc.toin.ac.jp
(Received September 27, 2004; Revised March 2, 2005; Accepted March 11, 2005)
Abstract
We determined photodynamic therapy (PDT) efficacy using a flash wave (FW) and a continuous wave (CW) light, of which the fluence rate was 70 µW/cm2, for murine thymic lymphoma cells (EL-4) cultivated in vitro. The irradiation frequency and the pulse width of the FW light were in the range of 1-32 Hz and less than one millisecond, respectively. 5- Aminolevulinic acid-induced protoporphyrin IX (ALA-PpIX) was used as a photosensitizer. When EL-4 with ALA administration was irradiated by the light for 4 h (irradiation fluence: 1.0 J/cm2 ), the survival rate of EL-4 by the FW light was lower than that by the CW light, except for the FW light with irradiation frequency of 32Hz, and decreased gradually with decreasing irradiation frequency. Moreover, the FW light, especially at lower irradiation frequency, was superior to the CW light for the generation of singlet oxygen in an aqueous PpIX solution. Therefore, the higher PDT efficacy for EL-4 of the FW light would be caused by the greater generation of singlet oxygen in the cells. ©2005 The Optical Society of Japan
Key words: photodynamic therapy, xenon lamps, flash wave light, continuous wave light, 5-aminolevulinic acid, protoporphyrin IX , oxygenation
1. Introduction
Photodynamic therapy (PDT) involves the administration and localization of photosensitizers to cancer tissues, followed by the activation of the photosensitizers by light with a specific wavelength. Photosensitizers excited by light transfer the energy to oxygen molecules, resulting in the formation of cytotoxic singlet oxygen. This therapy leads to a sequence of photochemical and photobiological processes that cause irreversible photodamage to cancer tissues. PDT is one of the most useful cancer therapies because of its low side effects and invasiveness, leading to an improvement in the “Quality of Life (QOL)” of patients.1) Hence, PDT has recently attracted much attention, and a great deal of biochemical and clinical research on PDT has been reported.2-6)
Light sources for exciting photosensitizers play an important role in improving PDT efficacy. PDT has often been carried out using laser light sources, including an argon ion laser, a diode laser, and a neodymiumdoped yttrium-aluminum-garnet (Nd:YAG) laser.1) In addition, pulse lasers are considered to be more effective than continuous lasers in providing a higher photon density into deeper cancer tissues and in destroying the tissues because of their high peak power pulses.7) It was previously reported that the PDT efficacy for mouse solid cancer 8) and rat brain cancer 9) developed in the deep tissues was higher by a pulse dye laser pumped using an excimer-laser than by a continuous dye laser pumped using an argon ion laser. The overall complete remission rate in PDT for stomach cancer with a pulse laser was also greater than that with a continuous laser.10)
PDT using laser light sources has the problem of the irradiation area of lasers being too small.11) In dermatology it takes a long time to irradiate laser light on an entire skin disease. To solve this problem, lamp light sources, including a halogen lamp, a xenon lamp, and a metal-halide lamp, have been employed instead of laser light sources, and outstanding clinical results were obtained.12-14) Nevertheless, almost all the lamps used in PDT employ continuous wave light, and there are few studies using lamps irradiating a flash wave light.
In the present study, we determined PDT efficacy for murine thymic lymphoma cells (EL-4), cultivated in vitro, with 5-aminolevulinic acid (ALA), using flash wave (FW) and continuous wave (CW) lights having the same fluence rate, and discussed the effectiveness of the irradiation frequency of the FW light on the PDT efficacy.
2. Materials and Methods
2.1 Light Sources
Flash wave (FW) and continuous wave (CW) xenon lamps (CF-40F, Nissin Erectronic Co., Ltd. and UI-501C, Ushio Inc.) were employed as light sources. The irradiation frequency and the pulse width of the FW light were in the range of 1-32 Hz and less than one millisecond, respectively. A UV and IR cut filter (Super Cold Filter 750, Asahi Spectra) and a red filter (302, OCLI) were used. The spectral output of the FW and the CW light was measured with a spectroradiometer (USR-40, Ushio Inc.).
2.2 Chemicals
5-Aminolevulinic acid (ALA, Cosmo Bio Co., Ltd.) and protoporphyrin IX (PpIX, Aldrich Co.) were used without further purification. ALA is an exogenous substance and a precursor of heme.15) When an excess amount of ALA is administered to cells, photosensitizing PpIX is formed and is temporarily accumulated in the cells.16) 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO, Aldrich Co.) was used as a spin trapping agent for measuring singlet oxygen. Histidine (Kanto Kagaku Co.) and dodecyltrimethylammonium bromide (DTAB, Nacalai Tesque Co.) were also used without further purification.
2.3 Cell culture
Murine thymic lymphoma cells (EL-4) were allowed to grow in an atmosphere of 95 % air and 5 % CO2 at 37 °C in RPMI 1640 medium containing 10 wt% fetal bovine serum (FBS) and a penicillin-streptomycin mixed solution. The cells were subcultured approximately twice a week.
2.4 PDT treatment
EL-4 cells were diluted with RPMI 1640 containing 10 wt% FBS to give a concentration of 5 x 105 cells/ml. A 3.5 ml of the diluted EL-4 dispersion was poured into 35 mm cell culture dishes. The diluted EL-4 dispersion was then incubated with 7 µl of 10 wt% ALA physiological saline for 3 h at 37 °C. The cells were exposed to light by the FW and/or the CW xenon lamp for 4 h. The survival rate of EL-4 was then measured with a cell viability analyzer (Vi-Cell, Beckman Coulter Co.). The survival rate was expressed as the percentage of the number of surviving cells against a blank, which was defined as the number of surviving cells that were incubated with the chemicals but not exposed to the light. The lower the survival rate was, the greater the PDT efficacy was.
2.5 ESR measurement of PpIX solution with irradiation of light
Five-tenths mg of PpIX was added to 50 ml of 0.1 mol/l aqueous DTAB solution, and the mixture was stirred with a shaker for 24 h and filtered through Millipore filter with 0.1µm pore size to remove excess PpIX. The aqueous PpIX solution was then diluted to 1 x 10-6 mol/l with 0.1 mol/l of aqueous DTAB solution. After 1 ml of 0.2 mol/l DMPO was added into 1 ml of 1 x 10-6 mol/l PpIX solution, the solution was exposed to light by the FW and/or the CW xenon lamp for 4 h. After the irradiation, the ESR spectra of the PpIX and DMPO mixed solution were measured with an ESR spectrometer (JES-AF200, JEOL). The ESR spectra of the mixed solution with histidine were also measured.
2.6 Statistics
Comparison of survival rates was performed using the t-test. Statistical significance (p) was less than 0.05.
3. Results and Discussion
Figure 1 refers to the spectral output of the FW (solid line) and the CW (dashed line) lights used in this study. We adjusted the irradiation distance from the lamps to obtain the same fluence rate. Although the spectral output of these lights was slightly different, their total fluence rate, which was calculated by the integration of the fluence rate in the range of 350-800 nm, was almost constant (70 µW/cm2). When estimating the absorption photon energy by PpIX for the FW and the CW light by the method described in our previous paper ,17) we confirmed that the same photon energy was adsorbed by PpIX in their irradiation. Moreover, the survival rate of EL-4 without ALA administration exposed to the FW and the CW light for 4 h was almost 100 %, indicating that neither light could directly damage the cells.
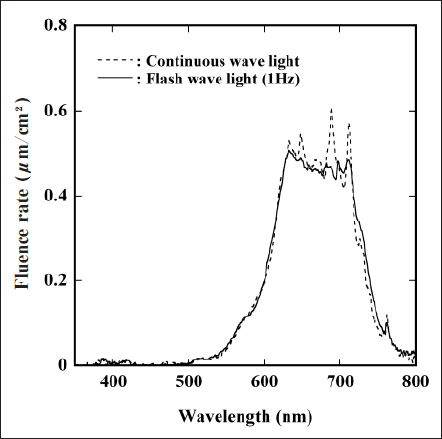
Fig. 1. Spectral outputs of FW light (solid line) and CW light (dashed line) used in this study.
Figure 2 shows the survival rate of EL-4 in the 4-h irradiation of the FW light combined with the administration of ALA. We also represent the survival rate in the irradiation of the CW light by the dashed line in this figure. According to the fluence rate and the irradiation time, the irradiation fluence of the light was 1.0 J/cm2. The survival rate in the irradiation of the FW light at 1-16 Hz was significantly lower than that, but at 32 Hz this rate did not differ from that of the CW light. In addition, the survival rate in the irradiation of the FW light gradually decreased with decreasing irradiation frequency. Therefore, the FW light, especially at lower irradiation frequency, is considered to promote PDT efficacy more than the CW light, despite having the same irradiation fluence.
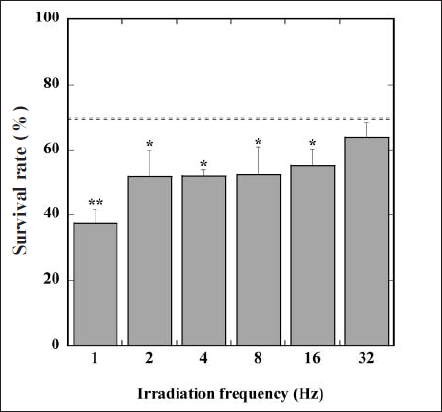
Fig. 2. Changes in survival rate of EL-4 in the presence of ALA with irradiation frequency of FW light. Values are mean ± SD. *and ** mean statistical analysis,p < 0.05 and p < 0.01 compared with survival rate in the irradiation of CW light (dashed line).
In PDT photoexited photosensitizers transfer the energy to molecular oxygen, forming singlet oxygen molecules (1O2). 1O2 can act as a cytotoxin and damages cells. Thus, the greater PDT efficacy of the FW light is thought to be related to the concentration of 1O2 generated in EL-4. We then observed the difference in the generation of 1O2 in an aqueous PpIX solution between the FW and the CW light irradiations using ESR spectroscopy with DMPO. When 1O2 is generated in a DMPO solution, DMPO generally forms DMPO-OH adduct, which gives the characteristic ESR signal having four peaks whose intensity ratio is 1 : 2 : 2 : 1.
Figure 3(a) shows the ESR spectrum of an aqueous PpIX solution in the presence of DMPO before the light irradiation. We could observe no ESR signal. When the PpIX and DMPO mixed solution was exposed to the FW light with 4 Hz in the irradiation frequency for 4h, we obtained an ESR signal characteristic of DMPO-OH adduct, represented in Fig. 3(b). Moreover, Fig. 3(c) refers to the ESR spectrum of the histigine-containing PpIX and DMPO mixed solution in the irradiation of the FW light. Histidine is well known as a quencher of 1O2 18). There was no ESR signal in this system, or in the system without the light irradiation. We also observed the same tendency in the ESR measurements with the FW light having the other irradiation frequencies and the CW light. Therefore, these results indicate that 1O2 was generated in the aqueous PpIX solution by the FW light and the CW light irradiation.
We further calculated the relative intensity (RI) value of the ESR signal of DMPO-OH adduct against the signal of manganese (Mn2+) marker on both sides of the ESR signals. Here, increasing RI value means increas ing generation of 1O2. Figure 4 depicts changes in RI value as a function of irradiation frequency of the FW light. The RI value in the irradiation of the FW light was larger than that of the CW light (dashed line); additionally, this value drastically increased with decreasing irradiation frequency. These results signify that the FW light is superior to the CW light for generating 1O2, especially at lower irradiation frequency.
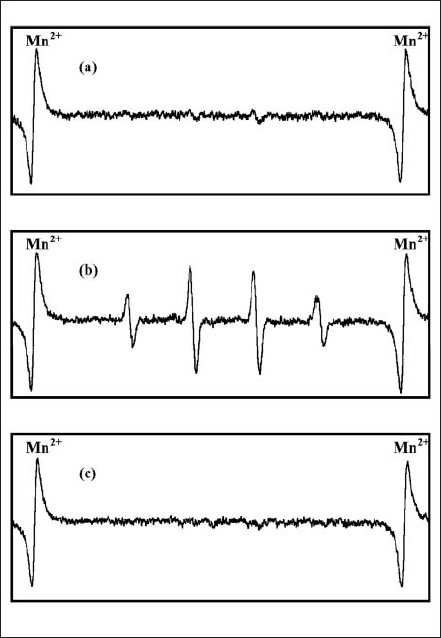
Fig. 3. ESR spectra of PpIX and DMPO mixed solution (a) before light irradiation; (b and c) after FW light irradiation (4Hz) for 4h without and with histidine.
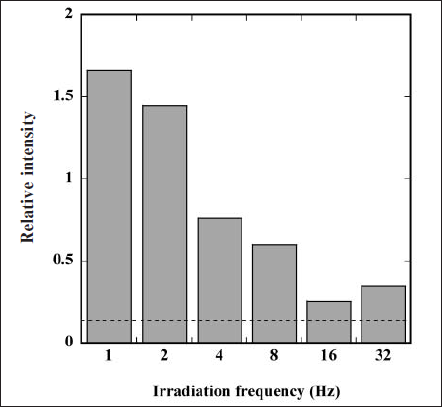
Fig. 4. Changes in relative intensity (RI) value of ESR signal of DMPO-OH adduct against the signal of manganese (Mn2+) marker as a function of irradiation frequency of FW light. RI value by irradiating CW light is also represented by the dashed line.
Curnow et al. investigated changes in partial pressure of oxygen in tissues in vivo by fractional light doses in PDT.19) They reported that the oxygen molecules in the tissues were obviously consumed to form 1O2 during the light irradiation, but that the partial pressure of oxygen rose again during the dark interval. In view of this change in the partial pressure of oxygen molecules in tissues in vivo during fractional light doses, our result on the greater 1O2 generation by the irradiation of the FW light is suggested to depend on dark interval compensation for the lack of oxygen molecules during the FW light irradiation, and the FW light having lower irradiation frequency can generate a larger amount of 1O2 by the compensation of oxygen molecules in the longer period of the dark interval.
When comparing the generation of 1O2 with the survival rate of EL-4 in the light irradiation, we conclude that the FW light, especially at lower irradiation frequency, causes the PDT efficacy for EL-4 to be greater than the CW light, because the generation of 1O2 in the cells is greater by the former light irradiation. This finding therefore confirms that the FW light irradiation is effective in promoting PDT efficacy without increasing irradiation fluence.
Acknowledgment
This study was partly supported by an Academic Fron-

