Part 1: The POCT Concept
Point of Care Testing (POCT) refers to conducting medical tests quickly on site at the patient’s location. To some, it might not sound so different from ordinary testing, but POCT offers a host of important advantages.
“Right There” and “Right Away”
These key concepts distinguish POCT from ordinary testing. Many people take the trouble to visit the hospital when they feel unwell, only to experience the disappointment of being asked to return at a later date to hear the results of their tests. With POCT, patients can hear the results of their blood tests or sample analysis right away. POCT is defined as testing conducted by the physician right beside the patient, using the advantages of shorter testing time and sense of closeness to the patient to contribute to swift and appropriate diagnosis, care, disease prevention, and health promotion, and ultimately to improving the quality of healthcare, and thereby the patient’s quality of life (QOL).*1
In other words, POCT is a kind of patient-friendly testing that doesn’t require returning to the clinic at a later date to hear results, or a lengthy wait in the waiting room. Common examples of POCT tests include the influenza tests conducted at hospitals and home pregnancy test kits.
*1 From the POCT Guidelines of the Japan Society for Clinical Laboratory Automation (translated into English by the Company)
Qualitative or Quantitative—That Is the Question.
Both the influenza tests and pregnancy tests are “qualitative” tests—they are “yes or no” style tests where the result is expressed as either positive or negative. There are already a wide range of commonly used reagents and testing techniques for such qualitative test subjects, and even at general hospitals, they can be handled with POCT-style tests.
However, to achieve increased QOL for patients and realize preventative therapies, there is a need for “quantitative” POCT that can accurately measure the concentration or amount of a certain substance and express it with high precision in numerical terms. Medical equipment manufacturers are competing to develop testing equipment and reagents to achieve this goal.
POCT Enables Patients to Start Therapy Right There, Right Away
Hepatitis B and C are said to be the largest epidemic in Japan today. Hepatitis C is particularly worrisome, since it often leads to sclerosis and then liver cancer when left untreated. Recently, there has been a lot of interest in phlebotomy therapy*2 in the treatment of hepatitis C. To determine whether this course of treatment is appropriate requires a quantitative test of the patient’s serum ferritin level*3, as the indicator.
Serum ferritin is commonly excluded as a blood test item during an ordinary health check. As a result, patients who require testing usually have to visit a major hospital that has the large and expensive equipment able to conduct a quantitative test. This has become an indirect factor preventing this effective method of therapy from becoming properly adopted on a wide scale.
In other words, because only a limited number of hospitals can provide the testing and therapy, many who are known to need it find themselves unable to receive it. Such people may include the elderly, the self-employed, and mothers busy with childrearing.
Furthermore, serum ferritin is also used as a marker for malignant tumors, and is used for diagnosing various cancers, including of the liver, pancreas, lung, and uterus. If there were a device or reagent at local clinics that could give an immediate test result, more people could be tested, enabling early detection of cancers in some cases. There are high expectations of POCT to provide such a means of early detection and subsequent treatment of diseases such as those that have remained hidden until now.
POCT in Developing Countries
The power of POCT goes beyond simply contributing to better QOL in advanced countries; it is also inherently easy for anyone to use, and delivers stable results at a low price. In developing countries there is a lack of expensive testing equipment able to perform quantitative tests, making it difficult for ordinary people to access them. This situation has created a need for simple, low-cost testing with test items customized to the conditions of each country or region, such as hepatitis C, which is caused by low standards of hygiene and medical practices, or endemic diseases such as thalassemia.
In response to these challenges, Japan is currently examining options for introducing testing equipment for POCT to developing countries, such as those in Southeast Asia, as a form of medical overseas development aid. The spread of POCT equipment will enable treatment and prevention of diseases through early detection, and help to raise healthcare standards and alleviate the economic burden. USHIO is contributing to this effort with its proprietary optical and imaging technologies.
| *2 | Phlebotomy refers to drawing blood from the body. Generally, this treatment involves drawing out between 200—400 ml of blood every 1—2 weeks in order to lower the serum ferritin level. The treatment can control the advance of hepatitis caused by excess iron in the body. |
| *3 | Ferritin is a protein that stores iron. Serum ferritin rises and falls reflecting the amount of iron stored in the body. Quantitative analysis of serum ferritin enables testing and diagnosis prior to phlebotomy therapy for anemia or hyperferremia, leukemia, and hepatitis C, and gives an insight into the state of the disease. Quantitative analysis is therefore generally in demand. |
Part 2: What Only Light Can Do
In April 2013, USHIO launched Japan’s first* blood analysis device capable of quantitatively analyzing serum ferritin by immunochromatography, the Point Reader®, and a dedicated reagent. The device makes use of USHIO’s optical and imaging analysis technologies, developed over many years.
*According to USHIO research as of March 31, 2013
What Is Immunochromatography?
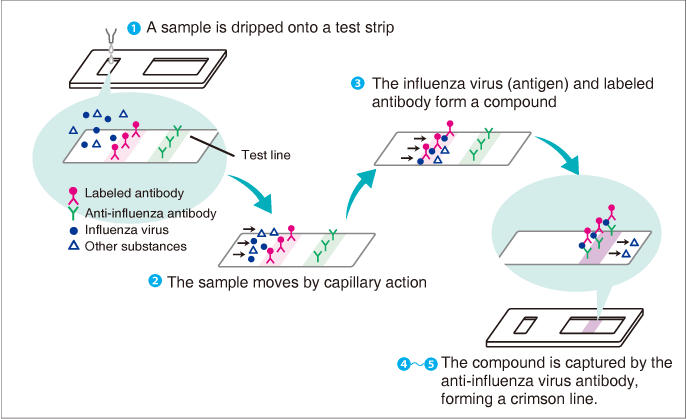
Immunochromatography is a commonly used testing method, found in familiar simple (rapid diagnostic) test kits such as for influenza and pregnancy tests. The word is taken from “immuno,” referring to the immune system, and “chromatography,” which means to separate a substance out into components. For example, in the influenza test, a sample taken from the patient’s nasal cavity is diluted and several drops of the solution dripped onto a strip ![]() . The sample then moves through the strip by capillary action
. The sample then moves through the strip by capillary action ![]() and if the influenza virus (antigen) is present in the sample it forms a compound with a labeled antibody
and if the influenza virus (antigen) is present in the sample it forms a compound with a labeled antibody ![]() . That compound moves further along the strip and is captured by an anti-influenza virus antibody on the test line
. That compound moves further along the strip and is captured by an anti-influenza virus antibody on the test line ![]() . The compounds emit a color that cannot be seen at the individual compound level, and as the number of captured compounds increases, the crimson (purplish-red) line acquires enough color to be seen by the human eye, allowing the tester to determine whether or not the virus is present
. The compounds emit a color that cannot be seen at the individual compound level, and as the number of captured compounds increases, the crimson (purplish-red) line acquires enough color to be seen by the human eye, allowing the tester to determine whether or not the virus is present ![]() .
.
Image of Measurement by Immunochromatography
Japan’s First System for Measuring Serum Ferritin by Immunochromatography

The Point Reader® and Point Strip®
Traditionally, the downside to the easy-to-use immunochromatography method has been its low level of precision in making quantitative measurements such as the percentage of a substance within a blood sample. The method has therefore been limited to tests where it is sufficient simply to show the presence of infection, such as influenza tests, and other qualitative analysis such as pregnancy or allergy tests.
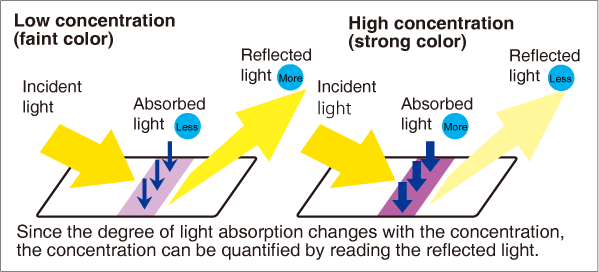
USHIO’s Point Reader® together with its dedicated reagent, the Point Strip®, are the first system in Japan to achieve quantitative measurements of serum ferritin using the immunochromatography method. The system uses proprietary optical technologies and algorithms to analyze fine changes in color intensity that depend on the concentration of serum ferritin in a blood sample. This represents a dramatic leap forward in the analysis capabilities of immunochromatography. The system shows excellent correspondence in results with those of large-scale automated analysis equipment, and opens the way for quantitative analysis of serum ferritin in a clinical setting that has previously been impossible. This breakthrough will eliminate variation in test accuracy and significantly relieve the burden of testing on both patients and physicians.
USHIO’s Proprietary Measurement Method
The needs for this kind of accurate on-site testing are multitude, including detection tests for mold, toxins, or harmful additives in food, for viruses and bacteria that cause infectious diseases, or for viruses biomarkers in the blood.
To meet these needs, USHIO is developing an on-site micro-analysis kit that uses a completely new proprietary measurement method called Q-body. A version of the device is currently being evaluated by the Japan Customs analysis division for use as a testing tool for import cargoes to crack down on drug smuggling.
USHIO will continue to work closely with people on various frontlines, applying its optical technologies to contribute to peace of mind, safety, and environmental protection for everyone.
Image of Optical Measurement
USHIO’s on-site microanalysis kit, currently being evaluated by the analysis division of Japan Customs