光技術情報誌「ライトエッジ」No.9(1997年2月発行)
Applied Physics Letters
Chemical surface modification on polytetrafluoethylene films vacuum ultraviolet excimer lamp irradiation in ammonia gas atmosphere
J.Heitz,a) H.Niino,and A.Yabeb)
National Institute of Materials and Chemical Research(MMC),Higashi 1-1.Tsukuba,Ibaraki 305,Japan
(Received 2 January 1996;accepted for publication 29 February 1996)
Irradiation of polytetrafluoroethylene films with vacuum UV light in an ammonia gas atmosphere resulted in a hydrophilic surface,where abstraction of fluorine atoms, and introduction of nitrogen,oxygen,and hydrogen atoms occurred. We used Kr2 * and Xe2 * excimer lamps at wavelength of 146 and 172nm,respectively.The reaction mechanism for chemical surface modification is discussed on the basis of x-ray photoelectron spectroscopy,secondary ion mass spectroscopy,and attenuated total reflection Fourier transform infrared spectroscopy analyses.c1996 American Institute of Physics.[S0003-6951(96)02419-9]
Many Promising new applications of polymers require specific surface properties in a thin layer, either at the whole surface or restricted areas thereon.Surface modification of polymers by photoinduced processes has been demonstrated for many polymers in recent years.1 However,fluorocarbon polymers having to significant absorption in the UV-(VIS) region are reluctant materials for conventional photochemistry,even though thermal surface damage can occur after high intensity irradiation with un UV excimer laser.2 Recently surface modification of polytetrafluoroethylene (PTFE) and other polymers by using UV laser irradiation in reactive media3-5 and vacuum UV (VUV) light treatment from a plasma discharge6.7 has been reported. In the past few years excimer lamps proved to be an inexpensive and simple light source for material processing.8-12 They can be operated in the VUV spectral region, where they can effectively decompose such inert molecules to UV-VIS light as NH3,N2O,O2,or H2O.From our previous results of ArF laser treatment of PTTE in hydrazine gas,3 we also expected PTFE surfaces exposed to photolyzed NH3 to show effective modification.
We used a Kr2* lamp(λ=146nm,Ushio Corp.,UER20H-146,head-on type)and Xe2* lamp (λ=172nm,UER20H-172,cylindrical type) with power densities between 10 and 20mW cm-2 at the lamp window. Excimer lamps emit light in the form of short pulses(several 10 ns FWHM) and are driven with high repetition rates of some KHz.13 The spectral bandwidths (FWHM) of Kr2* and Xe2* lamps were 12 and 14 nm in the emission spectra, respectively.13 The lamp was connected to a small vacuum chamber with a typical base pressure of 0.5 Pa,in which the samples were placed at a distance(D)of 1-10mm from the window. We irradiated PTFE samples(Nitto Denko Corp.,thickness:0.1-2mm)under continuous flow of NH3 gas.Contact angle measurement was carried out by using deionized water and 99.5% ethanol.Surface analyses were also performed by x-ray photoelectron spectroscopy(XPS;Perkin Elmer,PHI5600ci)using AlKα radiation,secondary ion mass spectroscopy (SIMS:PHI5600ci),and attenuated total reflection Fourier transform infrared spectroscopy (ATRFTIR ; Shimadzu, FTIR-4000, prism:KRS-5,detection angle:45°)
VUV irradiation of PTFE in NH3 gas resulted in a decrease of water and ethanol contact angle compared to nonirradiated samples,as shown in Table 1.The surfaces irradiated for 5 min and longer showed a visible color change from white to yellow/brown.When we investigated the irradiation of partially covered samples,no significant etching was observed by scanning electron microscopy.Figure 1(a) shows the dependence of the water contact angle on NH3 pressure(PNH3).At PNH3=4.0 Torr (526 Pa) the water contact angle reached its minimum value. An additional rinsing in water for 5 min in the ultrasonic bath resulted in an ~30°higher water contact angle [Fig.1(b)].Here we also found the minimum values of the contact angle at PNH3≈4 Torr.The modification time could be reduced by reducing D.In this case the minimum contact angles were found at higher value of PNH3 (D=1mm.PNH3≈10 Torr).The irradiation with the Xe2* lamp was less effective; i.e., 10 min irradiation at D=7 mm and PNH3=10 Torr(optimized value)resulted in a decrease of the advancing water contact angle to 40°without and to 70°after washing.
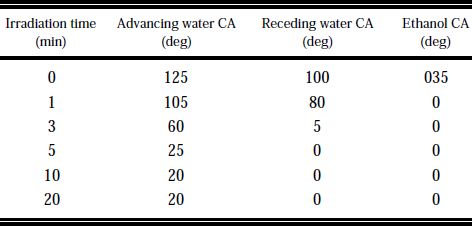
Table1 Advancing and receding water CA(contact angle) and ethanol CA on PTFE after treatment with VUV radiation in 4 Torr NH3 atmosphere (Kr2* lamp,distance of sample to window 7mm).
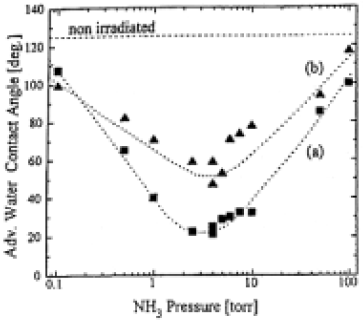
Fig.1. Dependence of water contact angle (advancing mode) on PTFE irradiated with a Kr2* lamp on the NH3 pressure during irradiation (irradiation time 5min, distance between sample and window 7mm). (a) Contact angle immedeately after orradiation, (b) additional rinsing in water.
Figure 2 shows the comparison of ATR-FTIR spectra of a nonirradiated sample and a sample irradiated for 90 min by a Xe2* lamp in NH3.In both cases strong C-F vibration peaks of PTFE dominate the spectral region below 1320cm-1.Abobe 1320cm-1 the irradiated sample showed a group of peaks(marked by their peak position).which can be assigned to the ammonium fluoride (NH4F) salt. It is water soluble and hygroscopic, and has a high vapor pressure.14 After rinsing the sample in water and drying in high vacuum, these peaks disappeared.Because the analytical depth of the ATR method is several µm, the small peak intensity of NH4F suggests that the thickness of the layer is in the nm range.
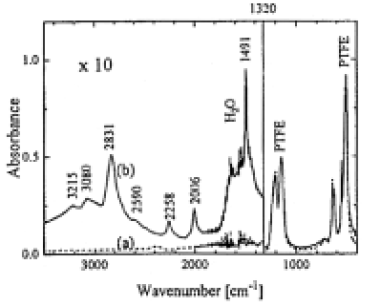
Fig.2. ATR-FTIR spectra of PTFE surface: (a) without irradiation,(b) irradiated for 90min by a Xe2* lamp in a 10 Torr NH3 atomosphere (distance between sample and window 1mm).The left part of the spectra is enhanced by a factor of 10.The background correction for H2O and CO2 is difficult for the ATR method, therefore the higher H2O peak intensity in spectra (b) is not necessarily due to a higher amount of H2O at the surface.
XPS investigation of the irradiated surfaces showed them to be altered when compared to nontreated samples.The relative content of F decreased strongly and incorporation of N and O occured(Table II).15 The samples were washed in water before XPS analysis, but we obtained similar spectra without washing.The fact that the summation of the relative intensities of F/C, O/C, and N/C results in value much smaller than 2, can be due to the formation of CH bonds(H is not directly accessible by the XPS method), or the formation of radicals or multiple bonds of carbons.From SIMS analysis (see below). and by the comparison with the experiments by ArF laser irradiation in a hydrazine atmosphere,16 we conclude that the first alternative is responsible for a considerable amount of the missing intensity.Fine scans of the C1S region show that on irradiated surfaces the shape of the peak changed(Fig.3). As indicated in the figure the maximum peak intensity shifted with increasing irradiation time from a spectral region assigned to CF bond to a spectra region, which can be assigned to either CO and CN bonds or to a CH bond.
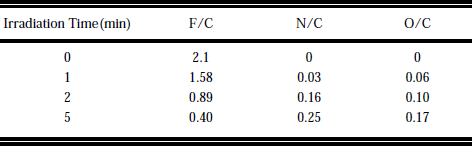
Table II. Atomic ratios deduced by XPS spectra of irradiated PTFE samples (Kr2* lamp,4 Torr NH3 atmosphere,distance between sample and window 1mm).
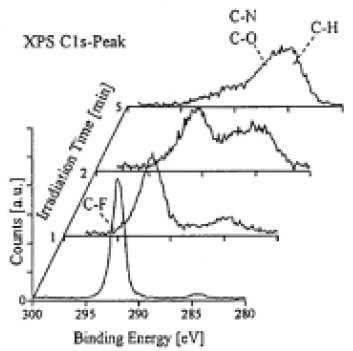
Fig.3.High resolution scan of the C1S peak of PTFE surface irradiated for different duration by a Kr2* lamp in a 4 Torr NH3 atomosphere (distance between sample and window 1mm).
Figure 4 shows the comparison of positive SIMS spectra of fresh and stronly modified PTFE samples. While fragments due to C+(mass number;12), CF+(31), CF3+(69),C3F5+(131), and other combinations of C and F were observed before irradiation, the spectrum after irradiation looked very similar to that of poly(ethylene), which is dominated by CnHm+ peaks(n=1-5,m=3-9).17The major peaks for PTFE are still present, but in much reduced intensity, and additional peaks correspond to fragments containing N atoms(at 18 amu NH4+ or at 30 amu CH2NH2+). These peaks are not present in the poly(ethylene) spectrum. For the irradiated sample the two main peaks of the negative SIMS spectrum were F- and CN-, but the absolute intensity of the F-peak was 6 times lower than that in the spectrum of nonirradiated PTFE.
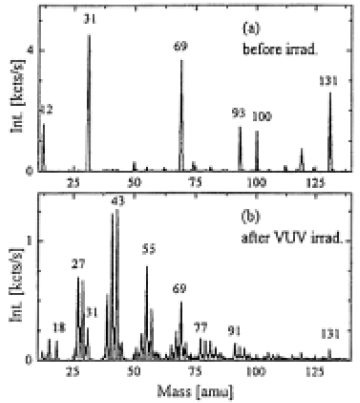
Fig.4. Positive static SIMS spectra of PTFE surface: (a) without irradiation,(b) irradiated for 90min by a Xe2* lamp in a 10 Torr NH3 atomosphere (distance between sample and window 1mm).
For λ below 220 nm the ammonia absorption spectra shows a series of absorption peaks, which are the vibrational regression of transitions to excited electronic states.18 The spectral bandwidth of the lamps covers several vibration peaks. The averaged absorption confficient is about 70cm-1 atm-1 for both lamps. Thus, in the case of the Kr2* lamp, more than 75% of the light reached the PTFE surface at optimized values of PNH3.The excited NH3 dissociates into H and NH2 with a quantum yield about 0.45 for the Kr2* lamp p and up to 1 for the Xe2 lamp.19 The difference between the H-NH2 dissociation energy of 4.3eV(Ref.20) and the photon energies of 8.5 and 7.2eV, respectivery, was transferred to the dissociation products.We assume that the H atoms, which could have high kinetic energy, reacted at the PTFE surface with CF groups to form carbon radicals in the surface and volatile HF molecules. Model calculations of this reaction on C5F12 using semiempirical methods showed it to be exothermic(ΔH=-23.28 kcal/mol, activation energy 6.11 kcal/mol).16 HF molecules reacted with the ammonia to form NH4F, which was at least partially redeposited on the PTFE surface(Fig.2). The carbon radicals on the surface could serve as reaction sites for H and NH2 radicals, which oriented either from the gas phase or from photoproducts on the redeposited layer.Formation of C=C bonds between neighboring radicals could also occur, but seems to be less likely.The oxygen on the surface could originate from contamination in the chamber,i.e., by water vapor, but more likely introduction of oxygen took place in air after removal of the sample from the chamber, by saturating the remaining carbon radicals or replacing other groups on the surface. The salt layer on the surface could be washed off in water, resulting in an increase of water contact angle as observed experimentally. In the XPS apparatus the layer disappeared under the ultrahigh vacuum conditions(10-9 Torr) employed there, due to its high vapor pressure.
Direct VUV irradiation of the polymer surface was necessary for modification, because the samples which were placed at an incidence angle of 90°were nearly not modified by exposing them to the photolyzed NH3. Thus a photoinduced process on the PTFE surface must be involved. We assume that this process is mainly due to photodissociation in the redeposited layer but up to now we cannot exclude other possibilities such as photoinduced temperature rise on the surface or the occurrence of excited states either in PTFE or in the modified surface layer due to the VUV irradiation.
We wish to thank the Ushio Corp.for the use of the Kr2 lamp and thank the NItto Denko Corp. for the polymer for the samples.


