光技術情報誌「ライトエッジ」No.31
2008.6 25th International Conference of Photopolymer Science and Technology (ICPST-25)
(2008年10月)
Techniques for Measuring Rate Constants for Acid
Generation from PAG (Photo Acid Generator)
during ArF Exposure
Atsushi Sekiguchi, Yoshiyuki Kono, Fumihiko Oda*1 and Yukihiro Morimoto*1
Litho Tech Japan Corp., 2-6-6 Namiki, Kawaguchi, Saitama 332-0034, Japan
Ushio Inc*1, R&D Center, 1194 Sazuchi, Bessyo, Himeji, Hyogo 671-0224, Japan
We previously performed in situ FT-IR measurements while subjecting chemically-amplified resists to UV light (248 nm) to observe photodecomposition of the PAG for KrF excimer lasers. Based on these observations, we measured rate constants for acid generation associated with PAG photodecomposition. More recently, we equipped our FT-IR system with a 193-nm excimer lamp (developed by Ushio Inc.) to perform the world's first in situ observations of PAG photodecomposition using 193-nm light. Our report also describes the successful measurement of rate constants for acid generation from the PAG for ArF excimer lasers.
Keywords
Chemically-amplified resist, FT-IR spectrometer, Photo Acid Generator (PAG), Rate constant
1. Introduction
Introduced in 1987 in a study by Ito et al.[1], chemically-amplified resists based on acid catalysts have become essential to the manufacture of semiconductor devices at sub-half-micron or smaller scales. Various studies to date have investigated improvements in resolution and environmental stability provided by chemically-amplified resists[2-4]. In a chemically-amplified positive resist, the Photo Acid Generator (PAG hereafter) generates acid in a photochemical reaction. This acid then works as a catalyst to remove protective groups in the Post Exposure Bake (PEB hereafter) following the exposure[1]. For this reason, it is important in the development of the resist and in the optimization of the processes to grasp the precise nature of acid generation from the PAG during the exposure. Based on this background, in several of our previous studies, we analyzed acid generation from the KrF resist during exposure[5-8], discussing results obtained with an FT-IR spectrometer equipped with a KrF exposure lamp. In this article, we modified the FT-IR spectrometer and equipped it with an ArF excimer lamp. We then proceeded to analyze the acid generated from the PAG with an ArF resist.
To observe only acid generation from the PAG, we added triphenylsulfonium-trifluoromethane-sulfonate (TPS-OTf hereafter) as the PAG to tricycle [5.2.1.02.6]-8yl metacrylate (TCDAMA hereafter) not containing protective groups. We prepared samples containing the PAG at resist ratios of 0%, 2%, 4%, and 8% and attempted to observe the acid generation reaction.
2. Hardware configuration
Fig.1 is a photograph of the experimental equipment used in this experiment. To measure thin film resists, the wafer was attached at 45 degrees with respect to the measuring IR light. The 193-nm excimer light was introduced from above. Attaching the wafer at 45 degrees relative to the measuring IR optical path increases measurement sensitivity[9-10].
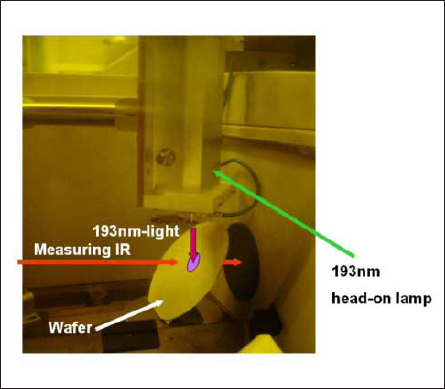
Fig.1: External appearance of FT-IR system equipped with ArF excimer lamp
A lamp emitting 193nm monochromatic light used for the experiment was an ArF excimer lamp developed by Ushio inc[11]. Fig.2 is a schematic of the ArF excimer lamp system, which was composed of the lamp and a power supply.
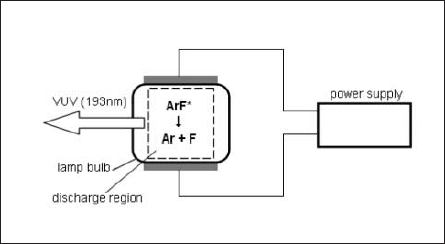
Fig.2: Schematic diagram of ArF excimer lamp
The ArF exciplex (ArF*) generated by the pulsed discharge emits 193nm-photons due to the transition from the exciplex state to the ground state. The radiation was taken from one end of the tube, whose spectrum is shown in Fig. 3.
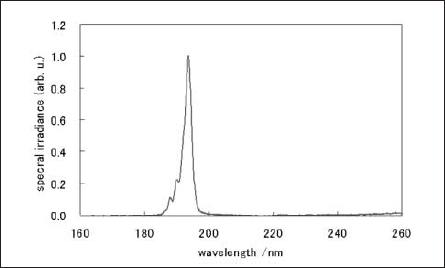
Fig.3: Spectrum of ArF excimer lamp
The spectral width (FWHM) of 193nm radiation band was about 3nm in this experiment. The irradiance and stability at the sample surface were 0.8mW/cm2 and < 10%, respectively, estimated by using a VUV monitor (UIT-150/VUV- S172, Ushio Inc.).
3. Experiment and results
To observe only acid generation from the PAG during ArF exposure, we prepared model resists. We used TCDAMA, a monomer, as the base resin, in place of a polymer. We added TPS-OTf as the PAG to this base resin at resist ratios of 0%, 2%, 4%, and 8%. Configuring sample compositions in this way avoids the deprotection reaction during exposure and enables tracking of only acid generation from the PAG. Fig. 4 shows the structural formulae of the model resist.
The ArF exposure was performed at approximately 0.8 mW/cm2 at the wafer surface. Fig. 5(a) shows the difference spectra before and after exposures at various PAG concentrations. Fig. 5 (b) shows the change in the peak during exposure times at 1270 cm-1 (for PAG concentration of 8%). At 1270 cm-1, we can observe a decrease in fluoromethan sulfonily. (A report by J. V. Crivello et al. discusses acid generation with exposure of TPS-OTf [12].) Figure 6 shows the reaction scheme of the TPS-OTf photoacid generation reaction. The acid appears to form through direct excitation of the PAG and by a sensitization mechanism involving electron transfers induced by photons absorbed by the polymer matrix[12].
Fig. 7 shows the relationship between the exposure dose and the normalized acid concentration for the pre-bake temperature of 100°C. The normalized acid concentration is the acid concentration [H+] normalized with the value at an exposure dose of 1000mJ/cm2.
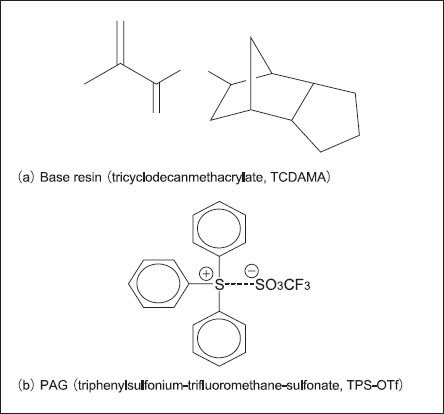
Fig.4: ArF model resist used in experiment
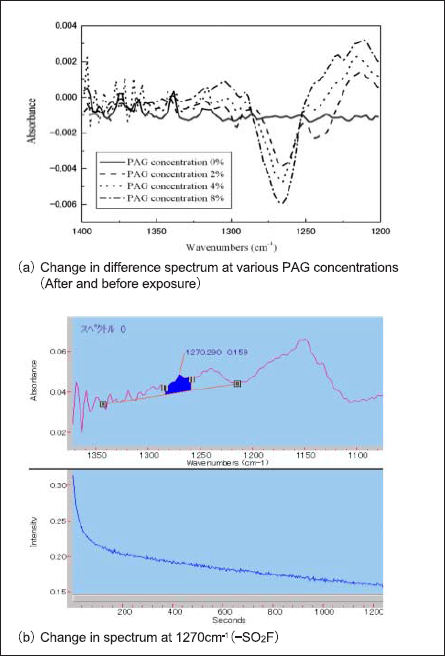
Fig.5: Comparison of difference spectra at various PAG concentrations and change in spectrum (1270cm-1)
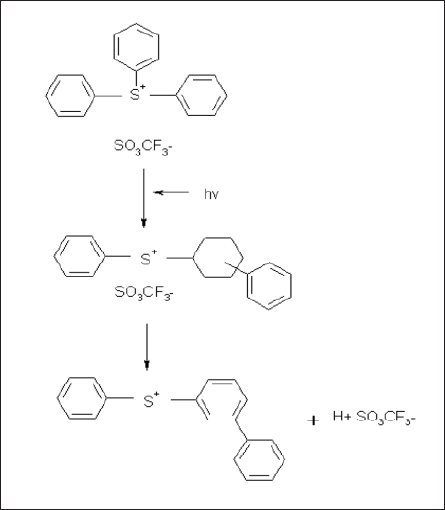
Fig.6: Photodecomposition of PAG
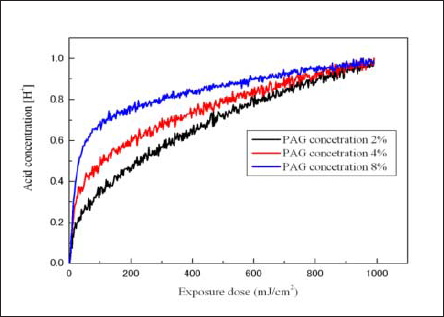
Fig.7: Relationship between normalized acid concentrations and exposure dose
4. Discussion
Table 1 shows the acid generation rate constant, C (Dill's C parameter), calculated by Equation (1), based on acid generation reaction curves, for pre-bake temperatures of 80°C, 100°C, and 120°C.
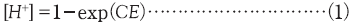
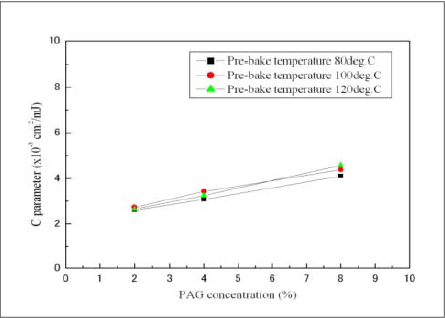
Fig.8: Relationship between C parameter and PAG concentration
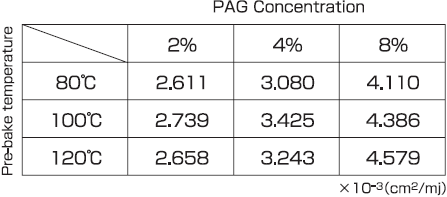
Table 1 : Relationship between PAG concentration and C parameter
The result shows pre-bake temperature does affect the C parameter, which increases as PAG concentration increases (Fig. 8).
In principle, the same PAG should have the same rate constant independent of PAG rate. According to the Spence model [13], acid concentrations after sufficient exposure should be the normalization value, 1, independent of the concentration of the generated acid. For this reason, larger PAG concentrations increase the amount of acid generated per unit exposure time and appear to increase the calculated apparent rate constant. We replotted the result using the concentration of the generated acid instead of the normalized acid concentration (Fig. 9). We adopted new model of photodecomposition by equation (2).
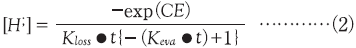
C is Dill's C, E is exposure dose, Kloss is reaction constants of acid loss, Keva is reaction constants of acid evaporation.
The slopes of the curves were approximately the same for all PAG concentrations.
Table 2 shows the acid generation rate constant, C, recalculated for the pre-bake temperatures of 80°C, 100°C, and 120°C. The values for the C parameter are approximately the same with the value about C = 0.004, independent of the pre-bake temperature and PAG dose.
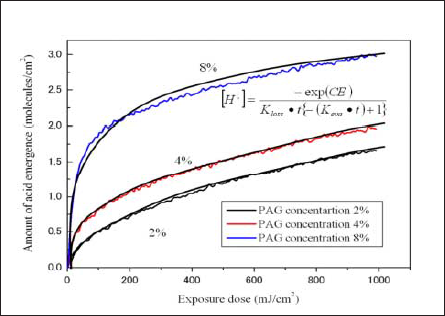
Fig.9: Relationship between concentration of generated acid and exposure dose
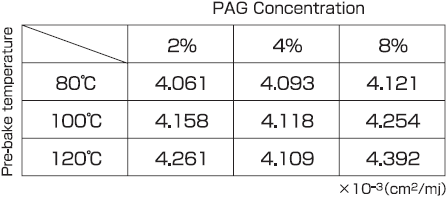
Table 2 : Relationship between PAG concentration and C parameter
5. Summary
We equipped an FT-IR system with a 193-nm excimer lamp and observed as acid was generated from PAG during ArF exposure.We used model resists to investigate reactions at PAG concentrations of 2%, 4%, and 8%, calculating Dill's C parameter. However, as we normalized the concentration of the generated acid with the value at the maximum exposure dose (normalization value = 1), the slopes of the curve differed for different concentrations and produced different C parameter values. In principle, the C parameter is an acid generation reaction constant and should be independent of PAG concentration. For this reason, we decided not to normalize the acid concentration, instead calculating the acid generation reaction constant based on the slopes of the curves for the relationship between concentrations of acid generated and exposure dose. We obtained a more or less constant value for the C parameter independent of PAB temperatures and PAG concentrations. We believe using this system facilitates the study of PAG materials and PAG rate.
Acknowledgments
We wish to thank We wish to thank Mr. Kusumoto and Mr. Ebata of the JSR Corporation Electronic Materials Research Laboratories for various materials and useful advice during the course of this research. We wish to thank Mr. Ogawa of TORAY Research Center for useful advice during the course of analysis FT-IR data.


