RadTech Asia
(2022)
Photochemical surface treatment of polytetrafluoroethylene at 172 nm with a Xe excimer lamp
A. Shimamoto*, and T. Fukuda*
*Ushio Inc., 1194, Sazuchi Bessho-cho, Himeji, Hyogo, Japan
Abstract
Photochemical surface treatment of polytetrafluoroethylene (PTFE) has been investigated by irradiation with UV light at 172 nm and 30 mW/cm2 under ethanol vapor conditions. After 2-min irradiation, the water contact angle of the PTFE surface decreased from 115° to 52°. The modification mechanism is discussed on the basis of the change of chemical composition of the PTFE surface, using XPS spectroscopy. It was found that the hydrophilic functional groups were generated on the PTFE surface, which is expected to improve the adhesion strength.
I. INTRODUCTION
Fluoropolymers are expected to be used for high frequency signal transmission applications because of their excellent electrical properties[1]. However, due to the high C-F bond energy, surface modification is difficult to improve their adhesion property. We have successfully modified the surfaces by cleaving the C-F bonds, using nascent H atoms produced by the photolysis of ethanol but not with water and methanol. Spectroscopic analyses have revealed the surface reaction mechanisms.
II. EXPERIMENTAL
A Xe excimer lamp (172 nm, 30 mW/cm2, USHIO Inc.) was used as a UV light source. The lamp and a polytetrafluoroethylene plate (PTFE, Yodogawa HuTech) were placed in a reactor with a 1-mm distance. High purity (>99.9995 %) nitrogen gas was bubbled into 15 mL of water, methanol or ethanol at a flow rate of 2L/min, purging the reactor. After the lamp was turned on for 30–120 s, the PTFE samples were removed from the reactor. The sample surfaces were analyzed by: (1) Water contact angle (WCA) measurement using a contact angle meter (Kyowa Surfaces & Materials, DMs-401), and (2) X-ray photoelectron spectroscopy (XPS, ULVAC-PHI, PHI Quantera II).
III. RESULTS AND DISCUSSION
Figure 1 shows the WCA changes of the PTFE surface as a function of treatment time. UV irradiation under ethanol vapor conditions decreased WCA from 115°to 52° after 120-s irradiation. We suspect that a nascent H atom from photolysis of ethanol adsorbed on the surface directly attacks the C-F bond to abstract the F atom as an HF molecule.
When water or methanol was used, WCA did not significantly change. This is because (1) Amounts of water and methanol adsorbed onto PTFE are too low for surface reactions to occur, and/or (2) Physical factors such as surface roughening increase WCA.
Figure 2 shows the XPS spectra of the PTFE surface before and after 120-s irradiation under ethanol vapor. XPS shows the chemical state of a few-nm depth of the surface layer. 120-s treatment removed almost all F atoms from the surface layer, forming a hydrocarbon layer that contains hydrophilic functional groups such as OH and C=O. The OH groups originate directly from the photolysis of ethanol. The C=O groups are generated by the oxidation of C–OH by O atoms or OH radicals, which are produced by the photolysis of a trace amount of residual O2 and H2O in the reactor.
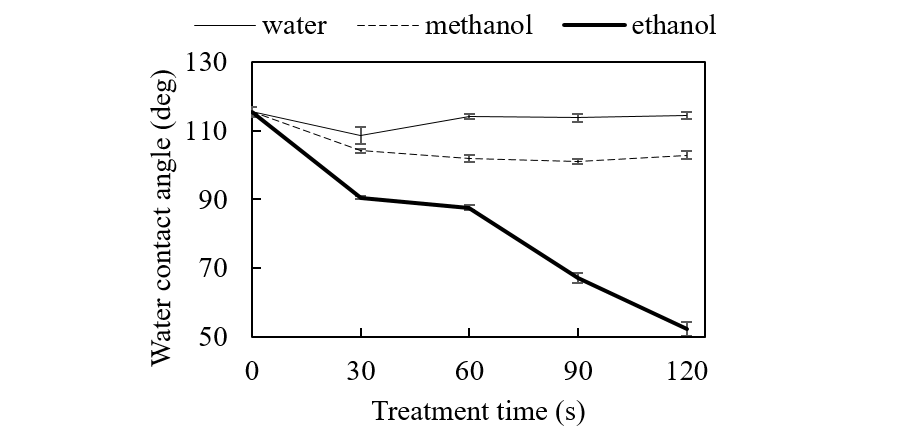
Fig. 1. The WCA of PTFE surface versus treatment time.
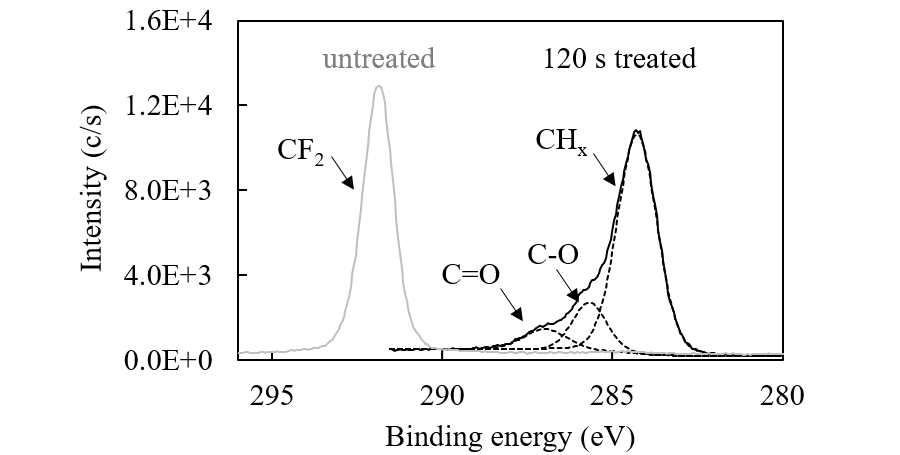
Fig. 2. The XPS spectra of the PTFE surface before and after 120-s UV treatment under ethanol vapor conditions.
IV. CONCLUSION
The PTFE surface was successfully hydrophilized by surface photoreaction using ethanol vapor. This process can be applied to a pretreatment for bonding fluoropolymers with other materials.
REFERENCE
[1] G. Primc, “Recent Advances in Surface Activation of Polytetrafluoroethylene (PTFE) by Gaseous Plasma Treatments,” Polymers 12 (10), 2295 (2020).
Copyright © USHIO INC. All Rights Reserved

