43rd International Symposium on Dry Process
Consideration of Reaction Mechanism by Calculation of
Behavior of Oxygen Active Species in Photodesmear®
Method
S. Endo1 , M. Miura2
1Ushio Inc., 6-1-1 Nishinakajima, Yodogawa-ku, Osaka, Osaka 532-0011, Japan
2Ushio Inc., 1194 Sazuchi, Bessho-cho, Himeji, Hyogo 671-0224, Japan
A dry process is being studied in the semiconductor packaging process. We have been studying the effectiveness of the Photodesmear® process using vacuum ultraviolet (VUV) light. By optimizing the conditions of high output ultraviolet light, high oxygen concentration, and temperature assist, we have achieved a very high process speed. We calculated the concentration of oxygen-active species in the Photodesmear® reaction atmosphere model for the effectiveness of shadow smear removal in photochemical reactions. Then, as a result of considering the reaction mechanism from the existence distance of each active species, it was found that the action of triplet oxygen is large.
1. Introduction
There is a smear at the bottom of the blind via holes formed using a CO2 laser. Smear is a substance in which the glass epoxy resin of the insulating layer is denatured by the heat of a laser. Smear must be removed from the bottom of the blind via holes to ensure electrical connectivity.
Generally, smear is removed by chemical treatment with a strong alkali or permanganate oxidizing agent. The wet chemical process has reached its limit for the miniaturization of the semiconductor packaging process.
It is said that the photochemical reaction using ultraviolet light and ozone depends on the high reactivity of O(1D) (singlet oxygen radical). Singlet oxygen has a short life and does not react in the shaded area where it is not exposed to light.

Fig. 1. Cross section image of blind via holes using Photodesmear® process.
The Photodesmear® process is established under the conditions of high power excimer lamp, high concentration oxygen, narrow gap, and temperature assist. In this Photodesmear® treatment, the removal of smear in the shadow portion of the blind via has been confirmed[1]. We considered the behavior of oxygenactive species in the Photodesmear® method.
2. Process and Simulation
2.1 Photodesmear® Process
The UV irradiation conditions for the Photodesmear® process in this study will be explained. The work was laminated with a glass epoxy resin (25um thickness) as an insulating layer on a copper-clad laminate. A blind via hole with a diameter of 50 microns was formed through the insulating layer using a CO2 laser. The surface of the blind via produced a smear of approximately 1 micron at the bottom.
A synthetic quartz plate was placed with a 300um gap from the work. Oxygen gas was flowed at a constant speed into the space between the work and the quartz plate. A Xe2 Excimer Lamp was placed on the opposite side of the quartz plate, and the work was irradiated with VUV light centered at 172 nm through the quartz plate.
Oxygen active species are generated by the photolysis reaction of oxygen by VUV light. Desmear progresses as oxygen active species react with smear. Furthermore, the reactivity is enhanced by heating the work together with light irradiation to raise the temperature of the entire system.
2.2 Simulation
In order to investigate the formation and extinction of active oxygen species by VUV light irradiation, a 0-dimensional photochemical reaction model was devised when a system consisting of oxygen and nitrogen was irradiated with VUV light.
A total of 26 reactions were considered, including photodecomposition of oxygen by VUV light and chemical reactions by oxygen active species.
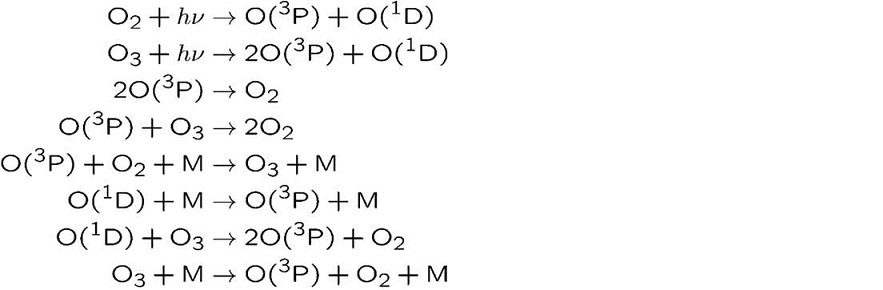
Fig. 2. The reaction model (partially) of the space above the work during VUV light irradiation.
O (3P): triplet oxygen, O (1D): singlet oxygen, and M is O2, N2, or O3. The reaction rate quoted the value described in the reference [2].
And, photochemical reaction rate constants k is defined below.
k = ∫ dλ σ(λ) I(λ)
where, λ is the wavelength, σ(λ) is the absorption cross section at λ, and I(λ) irradiation power.
In order to discuss which oxygen species are effective in removing smears at the bottom of the via, which is a shaded area where light does not reach, this model was used to calculate the change in the concentration of oxygen species after the light source was turned off.
All 0-dimensional calculations performed this time were performed using a reaction engineering simulator using an open-source Java kinetics package named Tenua [3].
3. Results and Discussion
This time, the system which reached the equilibrium state under irradiation of light source power 400mW/cm2 was taken as the initial state, and the change in the concentration of oxygen active species after the VUV light was turned off was calculated.
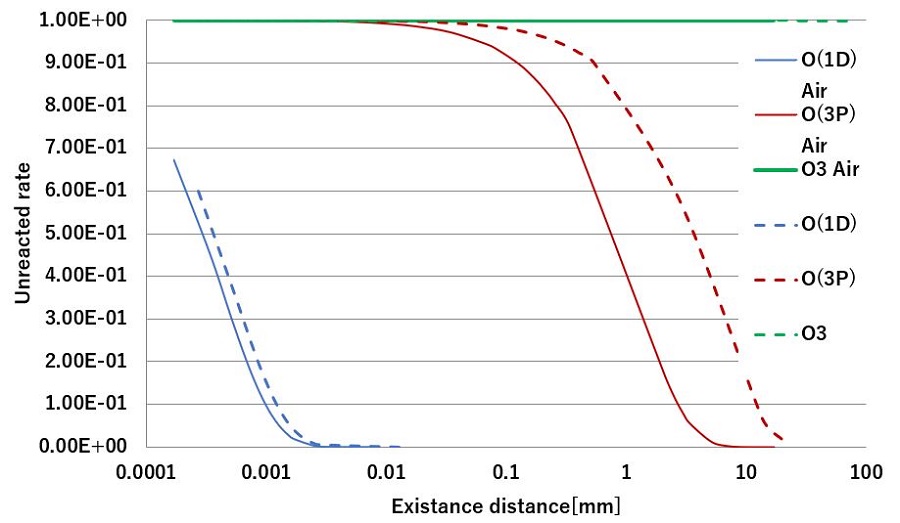
Fig. 3. The relationship between Existence distance and unreacted rate.
For comparison, the calculation was performed under two conditions: O2 100% 150℃. atmosphere and air (N2=80%:O2=20%) 25°C. atmosphere used as the Photodesmear® condition. Fig. 3 shows the calculated results. In addition, the lifetime is defined as the time from the moment when the concentration is turned off until the concentration is reduced to 1/100, and the Boltzmann velocity multiplied by is expressed as the existence distance (T: temperature, m: Molecular mass).

The results are shown in Table1. The possible distance of ozone in the room temperature atmosphere is extremely long, but it has become considerably smaller under Photodesmear® conditions. On the contrary, the possible distance of O(1D) and O(3P)increased. In particular, the increase in O(3P) is large.
Table. 1. Existence distance of Oxygen active species

Ozone is produced in large quantities from oxygen by VUV light in the Photodesmear® process. After that, ozone is decomposed at a high temperature to produce a large amount of O(3P).
According to Fig.3, O(1D) has a significantly short possible distance. This indicates that there is almost no movement even if O(1D) is generated by light. In other words, the O(1D) reaction occurs only when exposed to light.
O(3P) shows that it is possible to move several millimeters in Photodesmear® space. It is thought that this diffuses O(3P) to the shadowed part of the light and reacts with smear to enable desmear process[4].
4. Conclusion
The reaction of Photodesmear® shows higher decomposition action than in normal temperature atmosphere. The reaction mechanism that acts on the shaded area is expressed by the possible distance of the active species. The generation of a large amount of ozone by VUV light and the generation of triplets by temperature assist exceed the action of singlets.
References
[1] M. Namai, et al., “Full-sized Panel Photodesmear®for Via Residue Cleaning” in IEEE2016 Electric Components and Technology Conference, 2016
[2] N. Tabata, “Ozone generation and Generation Efficiency” J. Plasma and Fusion Research, 74-10, 1119 (1998)
[3] Tenua: The Kinetics Simulator for Java; http://bililite.com/tenua.
[4] A. R. Martin et al., “The Oxidation of Carbon Soot in a Non-thermal, Atmospheric Pressure Plasma: Experiment and Modeling” Journal of Advanced Oxidation Technologies, 8-2, 126 (2005)

