Surface reactions of silicon compounds by VUV-Reduction and VUV-Redox using a xenon excimer lamp
S. Endo, A. Shimizu
Ushio Inc.
A technology (VUV/O3 modification) is known that uses 172 nm vacuum ultraviolet light emitted from a xenon excimer lamp to modify the surface of a material. Organic matter on the surface of a material is removed by the action of high-energy vacuum ultraviolet light and active oxygen. However, the surface of a material modified with VUV/O3 is generally oxidized and becomes inactive. In this study, we report the results of an analysis of the surface reaction of silicon compounds using surface activation methods (VUV-Reduction and VUV-Redox) that do not use oxygen during vacuum ultraviolet irradiation, use reducing gas or redox gas.
1. Introduction
Organic matter on the surface of a material can be decomposed by using the 172 nm vacuum ultraviolet (VUV) light emitted by a xenon excimer lamp. Cleaning technology using vacuum ultraviolet light is called VUV/O3 cleaning and is used to clean the surfaces of glass substrates for liquid crystal panels and silicon wafers. The active oxygen (O*) generated by the VUV light acts on the organic matter (CxHyOz) on the surface of the material, and the organic matter is converted into carbon dioxide and water vapor and vaporized.
CxHyOz + O* → mCO2↑+nH2O↑ (1)
Plasma activation is used as a pretreatment for processing silicon wafer surfaces such as plating and bonding. However, there are concerns about plasma processes, such as long throughput due to the use of vacuum, difficulty in process control due to the difficulty of measuring plasma, and potential electrical damage to the workpiece.
J.X. Li et al. observed the reduction reaction on the copper surface by irradiating a copper sample, which had been heated in an oven at 235 °C for 15 minutes to form a thermal oxide film, with vacuum ultraviolet light in a reducing gas atmosphere [1]. Although it was previously thought that oxygen was required to use VUV light, the application of VUV light to reduction reactions using hydrogen was demonstrated.
We have developed a small irradiation device to replace the ambient gas on the sample surface when irradiating it with VUV light. Nitrogen mixed with 5 vol% hydrogen was used as the replacement gas when irradiating it with VUV light. The surface activation method using VUV light with reducing gas was called the VUV-Reduction method. In addition, before introducing the reducing gas into the small irradiation device, water vapor with a relative humidity of 50% was mixed with the reducing gas through a 30°C pure water bubbler to create 5% hydrogen-mixed wet nitrogen gas (hereafter referred to as redox gas). The surface activation method using VUV light with redox gas was called the VUV-Redox method. We report the results of surface analysis of silicon compound samples using VUV light surface activation method with reducing gas and redox gas.
2. Experiment (Detailed information-1)
The samples used in the experiment were as follows: Silicon dioxide: Corning alkali-free glass Eagle 10 mm x 10 mm x 0.7 mmt, silicon: ASONE single crystal silicon (one side polished) 10 mm x 10 mm x 0.5 mmt, silicon carbide: ASONE SiC substrate (one side polished) 10 mm x 10 mm x 0.5 mmt.
The sample was placed in a small irradiation machine with a stage temperature of 150°C and an irradiation gap of 0.3 mm, and the atmosphere was replaced by flowing reducing gas or redox gas at 67 cm3/min. For comparison, an experiment was also conducted in which oxygen gas was flowed.
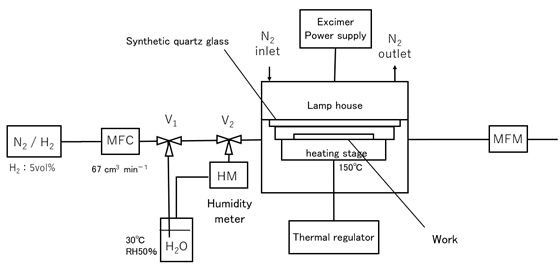
Figure 1. Configuration diagram of VUV light irradiation device
Then, VUV light was irradiated under the conditions of an illuminance of 330 mW/cm2 and an irradiation time of 10 seconds.
The surface composition ratios of carbon (C1s), oxygen (O1s), and silicon (Si2p) of the samples irradiated with VUV light in various gas atmospheres and the samples before irradiation were evaluated by a photoelectron spectroscopy (XPS).
3. Results and Discussion
3.1 Silicon dioxide
Silicon dioxide is a material that is widely used as a glass substrate, and there are many examples of VUV/O3 cleaning using oxygen. The C-C bond component at 284.6 eV, which showed a high peak when not irradiated, was significantly reduced by VUV/O3 treatment and VUV-Reduction treatment. The rate of reduction in 284.6 eV was small with VUV-Redox treatment.
3.2 Silicon
Figure 2 shows the C1s XPS spectra of silicon (Si) samples subjected to various VUV irradiation methods. Silicon is a material that is widely used as a semiconductor wafer, and there are many examples of VUV/O3 cleaning using oxygen. The C-C bond component at 284.6 eV, which showed a high peak when not irradiated, was reduced by half with various treatment methods using the same irradiation time. In terms of component ratio, oxidation was suppressed with the VUV-Reduction method, and the Si component ratio was the largest.
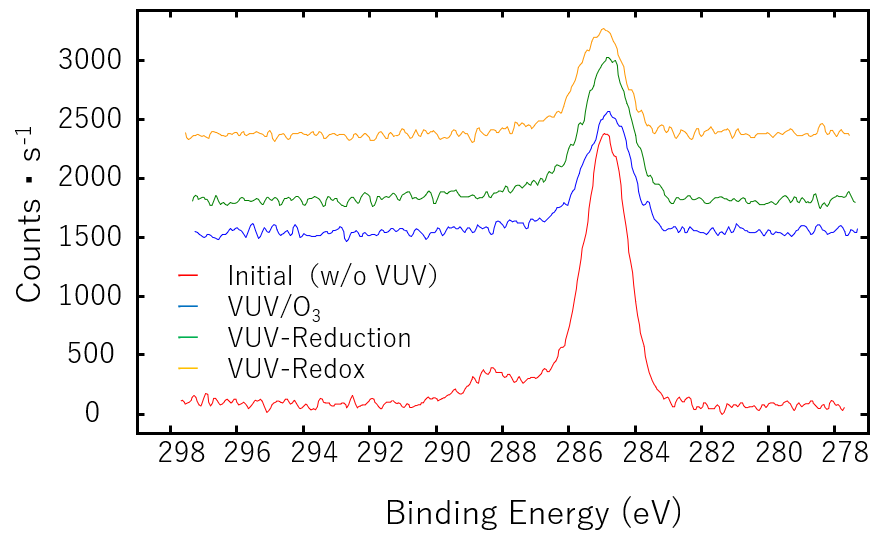
Figure 2. C1s XPS spectra of Silicon
Although there was no oxygen on the surface treated with the VUV-Reduction method, a phenomenon of carbon thought to be derived from organic components was observed. This is thought to be the effect of the reaction between the organic matter on the surface and hydrogen. In addition, the surface treated with the VUV-Redox method is thought to be affected by water vapor, which generates hydrogen radicals and hydroxyl radicals when exposed to VUV light, and it is thought that the action of these radicals promotes the evaporation of organic components.
H2O+ℎν (172 nm) → H・+・OH (2)
H2+・OH → H2O+H・ (3)
3.3 Silicon Carbide
Figure 3 shows the Si2p XPS spectra of silicon carbide (SiC) samples irradiated with various VUV methods. The Si2p component at 102.0 eV, which showed a sharp peak when not irradiated, showed a sub-peak of 104.0 eV when treated with VUV/O3 in an oxygen gas atmosphere. It is expected that a significant change has occurred in the Si bonding state under these conditions. No significant change was observed in the Si2p spectrum with the VUV-Reduction method. With the VUV-Redox method, the Si2p peak became higher than before treatment.
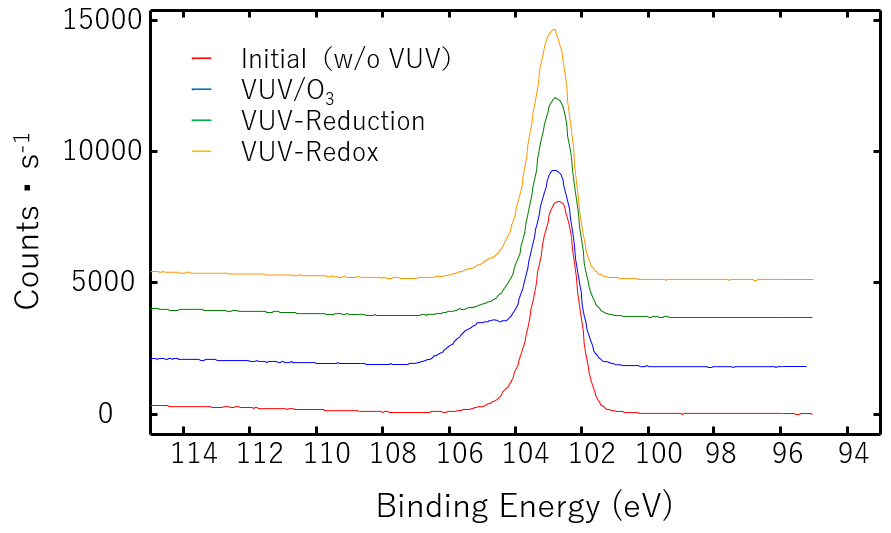
Figure 3. Si2p XPS spectra of Silicon carbide
The Si-C bond energy is 4.2 eV, which is lower than the 7.2 eV energy of 172 nm light. Differences in the absorption of energy from VUV light and the action of hydrogen radicals may be causing differences in the reactions on the SiC substrate surface.
4. Conclusion
We analyzed the surface of a silicon compound irradiated with VUV light in a reducing or redox gas atmosphere using XPS and confirmed a surface reaction different from that observed in conventional VUV light treatment in an oxygen atmosphere.
References
[1] J. X. Li et. al, Reduction of Copper Oxide Induced by 172 nm Vacuum Ultraviolet Radiation at Ambient Temperature, Journal of Electronic Materials, 40-10, 2105 (2011).
[2] I. Ohsawa et al., Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals, Nature medicine, 13, pages688–694 (2007)
[3] S. Endo et al, Activation of copper surfaces by VUV-Redox method using a xenon excimer lamp, The 89th Annual Meeting of the Society of Chemical Engineers (2024)

