光技術情報誌「ライトエッジ」No.24(2002年4月発行)
第50回高分子討論会
(2001年9月)
Nafion® Based Polymer Electrolyte Membranes For Improved
Direct Methanol Fuel Cell Performance.
Lois J. HOBSON1, Hideyuki OOZU1, Masanori YAMAGUCHI2and Shuji HAYASE3
1Corporate Research and Development Centre, Toshiba Corporation, 1 Komukai, Toshiba-cho, Saiwai-ku,
Kawasaki 212-8582, Japan.
2Ushio Inc., 1194, Sazuchi, Bessho-cho, Himeji, Hyogo 671-0224, Japan.
3Graduate School of Life Science and Systems Engineering, Kyushu Institute of Technology, 1-1 Hibikino,
Wakamatsu-ku, Kitakyushu 808-0196.
Tel. +81(0)44-520-2118, Fax. +81(0)44-520-1286, e-mail: hobson@sub1.rdc.toshiba.co.jp
Introduction
As fuel cells move swiftly into the commercial domain, with applications ranging from transportation to small portable electronic devices, the polymer electrolyte membrane is recognised as the limiting factor regarding exploitation of this technology. With specific reference to the direct methanol fuel cell (DMFC), where two electrodes separated by a polymer membrane form the core components, performance is largely dependent on the selective permeability characteristics of the membrane.1, 2
During operation methanol enters the system at the anode, where it is oxidised generating carbon dioxide [CH3OH + H2O →CO2 + 6H+ + 6e- ], while the corresponding reduction process takes place at the cathode, [4H++ O2 + 4e- →2H2O]. However, due to similar composition of the two electrodes, if methanol crossover through the polymer-electrolyte membrane is experienced then methanol oxidation can also occur at the cathode, dramatically reducing overall performance. Hence improvements in the selective permeability characteristic of the membrane, facilitating the passage of water but restricting methanol, can produce significant gain in terms of device performance.
One strategy to prepare materials with a high conductivity but low permeability to methanol is the modification of commercially available Nafion® film, creating a thin barrier layer at the surface, Figure 1. Whether modification is by chemical or physical means and is applied to one of both sides of the membrane the bulk of the material remains in tact, presenting the best chance of preserving the inherent conductivity of the parent material. Hence, this approach has been adopted, by us3-5 and others,6, 7 in application to fuel cell technology.
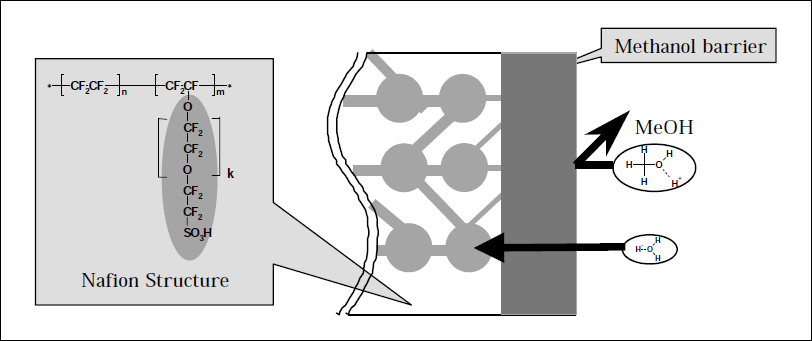
Figure 1. Nafion structure and schematic representation of proposed barrier strategy.
Experimental
Electron beam (EB) irradiation experiments were carried out using a low dose EB irradiation unit, MINEB-LAB from Ushio Inc. Japan. Typical irradiation conditions include an accelerating voltage of 35kV, with a tube current of 300µA/tube and scan speed of 3m/min (resulting in a dose/scan rate of 2.5µC/cm2 (uniformity +/-15%), carried out at <200ppm oxygen, EB penetration depth <10µm. DMFC operating conditions: a liquid feed system with no backing pressure, operating at 60°C, air flow 60ccmin-1 , methanol feed 0.6ccmin-1 .
Results and Discussion
We describe the use of low dose EB irradiation as a method to modify Nafion® surface structure, reducing the pore size of the hydrophilic region and hence changing the permeability characteristics of Nafion as illustrate in Figure 1.3 By this technique permeability to methanol was successfully reduced to a level approaching 50% of that recorded for the untreated film, whilst maintaining a conductance comparable to that of the parent material.
IR (ATR) spectroscopy was used to probe the effects of EB exposure on a molecularlevel, revealing generation of CO2H units at the site of the polymer backbone polymer and the concurrent loss of the Nafion® hydrophilic (- OCF2 - C(CF3) - F - )n -OCF2CF2 - SO3H side chain.4 Both mechanisms can be shown to be a function of irradiationdose and have opposing effects regarding the physical properties highlighted for FC technology. Hence, a complex relationship with respect to irradiation dose is expected (figure 2), making EB exposure a potential tool to prepare materials with the rare combination of low methanol permeability and high conductivity. These observations are supported by the physical properties of the bulk materials.
In terms of targeting the development of films for polymer electrolyte membranes, two key regions of EB exposure dose can be highlighted as producing materials of potential interest for DMFC technology; irradiation levels of ~10µC/cm2 and 100-200µC/cm2 . Figure 3 shows Power/Current density profiles for Nafion117 films before and after EB exposure at 9.3µ C/cm2 . The results shown are presented only as comparative data (no optimisation of operating conditions). Increased performance, in terms of both power output and operational current density range is observed; the effect of modification being more pronounced as we move towards fuel systems with a higher methanol concentration.
Similar results are shown for the surface modification of Nafion117 by chemical means; screen printing with dilute polybenzimidazole/ dimethylacetamide solutions was identified as one method to prepare materials exhibiting high conductance and reduced methanol permeability.5 Therefore we conclude that the barrier strategy described provides one of the most promising routes to polymer electrolyte membranes, facilitating combination of the properties required for direct methanol fuel cell operation.
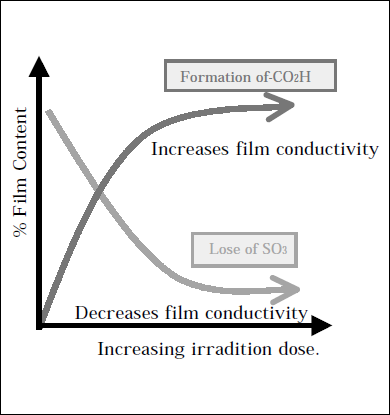
Figure 2 Balance of physical properties.
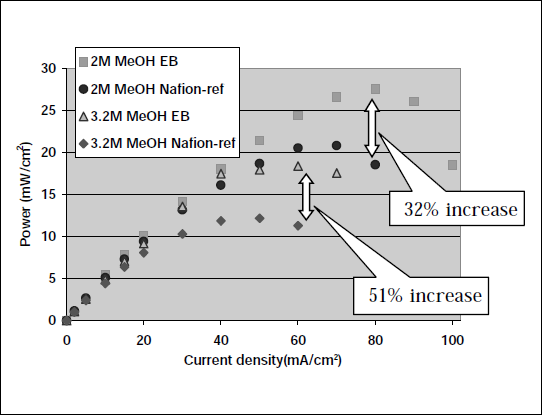
Figure 3 Comparative DMFC performance of EB treated films (9.3µC/cm2 ) and Nafion®117.


